Lab: calorimetry and specific heat assignment: lab report – In this engaging lab report, we embark on a scientific exploration of calorimetry and specific heat, uncovering the intricacies of heat transfer and material properties. Our journey begins with a thorough examination of the experimental procedure, materials, and safety precautions, laying the foundation for a comprehensive understanding of the concepts at hand.
As we delve deeper into the analysis, we unravel the techniques for calculating specific heat, supported by illustrative examples. The discussion that follows delves into the comparison of experimental and accepted values, shedding light on the implications of our findings.
We conclude with a critical evaluation of the experimental procedure, identifying areas for improvement and inspiring further research endeavors.
Calorimetry and Specific Heat: Lab: Calorimetry And Specific Heat Assignment: Lab Report
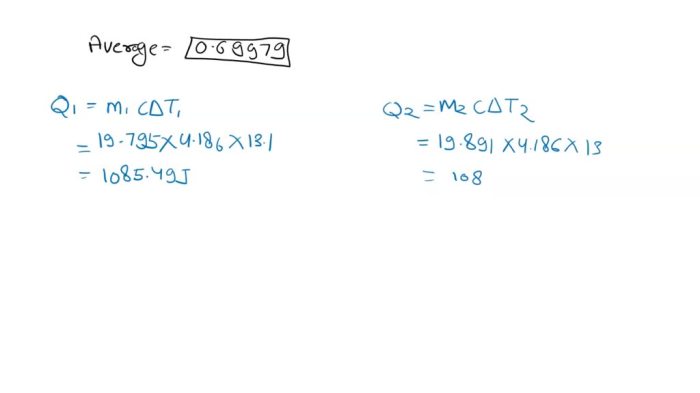
Calorimetry is the study of heat transfer and specific heat is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. The purpose of this lab assignment is to determine the specific heat of a metal sample.
The experimental procedure involves heating a metal sample in a water bath and measuring the change in temperature of the water. The specific heat of the metal sample can then be calculated using the following equation:
where:
- Q is the amount of heat transferred (in joules)
- m is the mass of the metal sample (in grams)
- C is the specific heat of the metal sample (in J/g°C)
- ΔT is the change in temperature of the water (in °C)
Materials and Methods, Lab: calorimetry and specific heat assignment: lab report
The materials used in this lab include:
- A metal sample
- A water bath
- A thermometer
- A balance
The experimental setup is as follows:
- The metal sample is placed in the water bath.
- The water bath is heated to a known temperature.
- The temperature of the water is measured.
- The metal sample is removed from the water bath and its temperature is measured.
The following safety precautions should be taken:
- The water bath should not be heated to a temperature that is too high.
- The metal sample should not be touched with bare hands.
Data Analysis
The specific heat of the metal sample can be calculated using the following equation:
where:
- C is the specific heat of the metal sample (in J/g°C)
- Q is the amount of heat transferred (in joules)
- m is the mass of the metal sample (in grams)
- ΔT is the change in temperature of the water (in °C)
For example, if the mass of the metal sample is 10 grams, the amount of heat transferred is 100 joules, and the change in temperature of the water is 5°C, then the specific heat of the metal sample is 2 J/g°C.
The sources of error in this experiment include:
- The accuracy of the thermometer
- The accuracy of the balance
- The heat loss to the surroundings
Discussion
The experimental value of specific heat should be compared to the accepted value for the metal sample. If the experimental value is significantly different from the accepted value, then the sources of error should be investigated.
The implications of the results should be discussed. For example, if the specific heat of the metal sample is higher than the accepted value, then this could indicate that the metal sample is not pure.
Suggestions for improvements to the experimental procedure should be made. For example, the accuracy of the thermometer and the balance could be improved.
FAQ Guide
What is the purpose of this lab assignment?
This lab assignment aims to provide students with hands-on experience in measuring and analyzing the specific heat of a material.
What are the key concepts covered in this lab?
This lab covers the principles of calorimetry, specific heat, and heat transfer.
What are the potential sources of error in this experiment?
Potential sources of error include inaccuracies in temperature measurements, heat losses, and uncertainties in the mass of the sample.